
medical electrical equipment is a medical device used for the diagnosis, treatment and monitoring of patients, as well as for the elimination or alleviation of disease, injury or disability. with the rapid development of science and technology and the continuous improvement of people's living standards and health care awareness, the application scope and field of medical electrical equipment are becoming more and more extensive, and the control of its use risk and the improvement of quality are becoming more and more important.
the quality control of medical devices has always been the focus of national service.
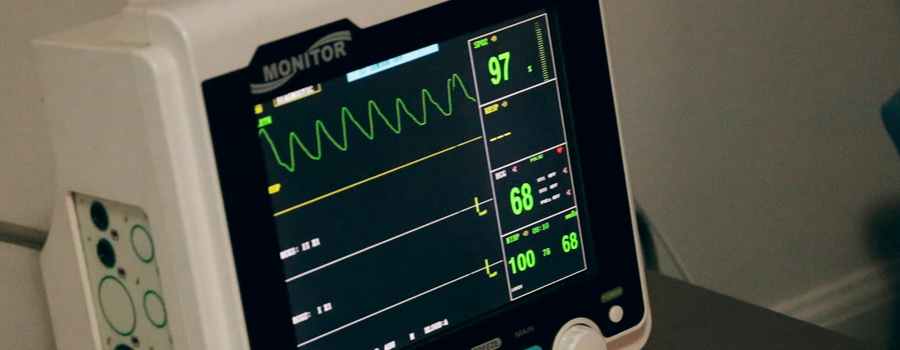
noa focus on the compliance and quality of electrical and electronic products, and establish a one-stop service platform for consulting, testing and certification. we provide professional testing services for medical electrical equipment. including active surgical instruments, ecg measurement and analysis equipment, physical therapy instruments, etc.
○ gb 9706.1/iec60601-1《medical electrical equipment—part 1: general requirements for basic safety and essential performance》
○ gb 9706.4/iec 60601-2-2《medical electrical equipment - part 2-2: particular requirements for the safety 0f high frequency surgical equipment》
○ gb 9706.25/iec 60601-2-27《medical electrical equipment part2:particular requirements for the safety of electrocardiographic monitoring equipment》
○ yy 0505/iec 60601-1-2《medical electrical equipment—part 1-2:general requirements for safety—collateral standard: electromagnetic compatibility—requirements and tests》
○ yy 9706.102/iec 60601-1-2《medical electrical equipment—part 1-2:general requirements for basic safety and essential performance—collateral standard: electromagnetic compatibility—requirements and tests》
○ gb 4824/cispr11《industrial, scientific and medical equipment—radio-frequency disturbance characteristics—limits and methods of measurement》
○ gb 4793.1/iec 61010-1《safety requirements for electrical equipment for measurement,control,and laboratory use - part 1: general requirements》
○ gb 4793.2/iec 61010-2-032《safety requirements for electrical equipment for measurement,control,and laboratory use - part 1: general requirements》
○ gb 4793.5/iec 61010-2-031《safety requirements for electrical equipment for measurement, control, and laboratory use - part 5: safety requirements for hand-held probe assemblies for electrical measurement and test》
classⅰ, classⅱ, classⅲ are classified according to the use safety of medical devices.
classⅰ is the medical devices with low risk, which can be guaranteed safe and effective by routine management.
classⅱ is a medical device with moderate risk, which needs strict control and management to ensure its safety and effectiveness.
classⅲ is a medical device with high risk, which requires special measures to be strictly controlled and managed to ensure its safety and effectiveness.
for safety protection, gb4793.1 is a protection requirement mainly for operators and the surrounding environment. gb9706.1 has a patient application part in addition to the operator and the surrounding environment, and also clearly puts forward specific requirements for the accuracy of work data and the prevention of dangerous output.






tel: 86-400 821 5138
fax: 86-21 3327 5843
email:noa@noagroup.com